20 miliardi di euro l’anno: i costi per il cancro in Italia – Prevenzione attiva, la vera arma vincente
Was awarded for “yearly monitoring the occurrence of cancer-associated somatic mutations in asymptomatic patients and to detect solid cancer in the prodromal stage i.e., before any clinical manifestation”.
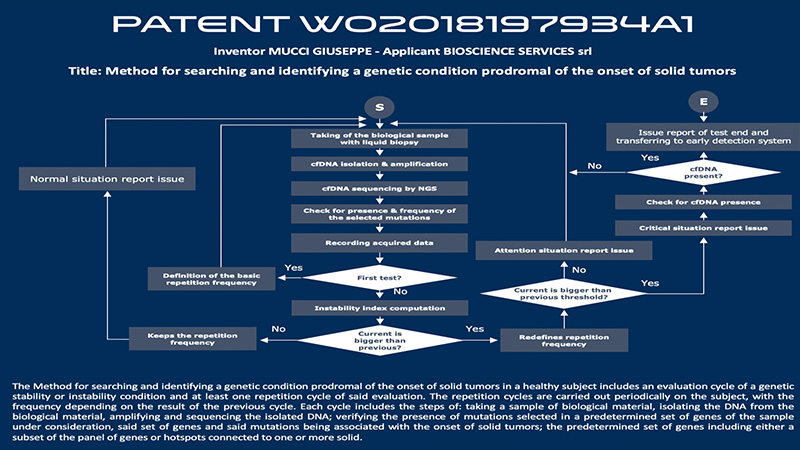
It is discovered that it might take 5 -15 years between the appearance of a first mutation and the actual development of cancer. Current diagnostic methods (such as mammography, X-rays, colonoscopy, and others) detect cancers when already formed. The first step of the patented algorithm is the analysis of certain genes that are involved in the maintenance of genetic stability, such as encoding factors involved in DNA damage response.
If mutated, such genes work as cancer driver factors. Identifying the cancer-associated mutations as soon as they occur and before the tumor is detectable with current diagnostic methods may enable the full eradication of the disease and significantly improve outcomes for the patient. Other genetic tests aimed at cancer prevention, which use somatic mutations, aim at the early diagnosis of cancer. However, it has been shown that the use of somatic mutations for early detection is not scientifically sustainable. The HELIXAFE Patent is, in fact, focused on the cancer prodromal stage and not cancer early detection.
A trademark of logo that identifies and distinguishes HELIXAFE and the Company is registered. We work with an IP attorney and an IP engineer who helps identify IP worth protecting, navigate the registration process, and provide guidance on enforcing our rights and filing patents and trademarks in other countries. The IP attorney already implemented contractual protections with employees, partners, and third parties, such as non-disclosure agreements (NDAs) and non-compete clauses, which can help safeguard your IP. The countries where the HELIXAFE patent has been granted are Italy, Japan and Korea. The rest of the world is still pending. The logo of the US Newco has already been registered.

Clinical, non-clinical, technological and industrial data will be collected throughout the project. Confidential and sensitive data related to the AI tool and participants in the clinical trials will be kept restricted and will not be published. Non-sensitive publishable data will possibly be shared through publications and made accessible to the research community for verification and re-use and for cross-validation scientific purposes. The collected data will be primarily used to develop the AI-based tool, IP protection further, and results dissemination and communication to interested stakeholders, including the scientific community, key opinion leaders, patients and the wide public. Data from clinical studies will be public and accessible from the University Hospital of Rome Tor Vergata (IT). We will value our IP through direct commercial exploitation of HELIXAFE tests and further analysis of the customer’s data through the optimized AI algorithm. The annexe reports the granted patent on the methodology to individuate cancer drivers in the prodromal phase of solid tumours. The patent ensures that there are no legal constraints due to third-party patents that could prevent the commercialization of our services.

